Quality Management System Framework
Richtek defines the procedures and instructions from product planning, development, verification, release, to production management including manufacturing quality and product conformity management. In response to the quality objectives, Richtek takes actions such as audits, voice of customers, and the internal performance review for continuous improvement. The following are Richtek Quality and Reliability Framework diagram and detailed descriptions.
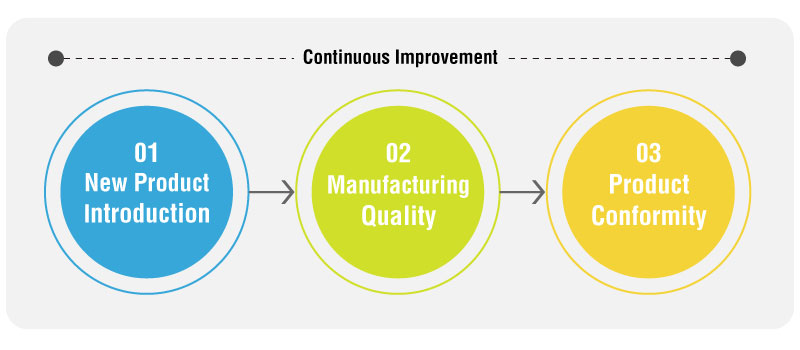
1. New Product Introduction:
Richtek researches the industrial and customer application requirements to define our product specification, and the specification is verified throughout the development, qualification and release stages.
Once the product has been reviewed for conformity to specifications and undergone a safe launch, it can be approved for mass production.
2. Manufacturing Quality:
As a fabless company, Richtek establishes a series of supplier management standard procedures and requirements for quality assurance, including new supplier selection, new process verification, material specification, control plan, change management, non-conforming handling, business contingency plan, regular supplier review, and audit programs.
3. Product Conformity:
Richtek performs final tests to products to ensure conformity to the specification; besides, the ongoing reliability tests are also carried out for quality monitoring; and the compliance status to green product regulations is also assured.
4. Continuous Improvement:
Richtek reviews quality performance, customer feedback, supplier evaluation result and internal audit result. We track the fall-behind items to implement the improvement actions as part of our continuous improvement program.